Introduction to Rapid Diagnostics
Rapid diagnostics refers to medical diagnostic techniques that can produce results in a very short period, often within minutes or hours rather than days or weeks. This allows for quicker clinical decision making and faster treatment. Some common types of rapid diagnostics include point-of-care testing (POCT), lateral flow assays, and PCR-based molecular testing.
Point-of-care Testing (POCT)
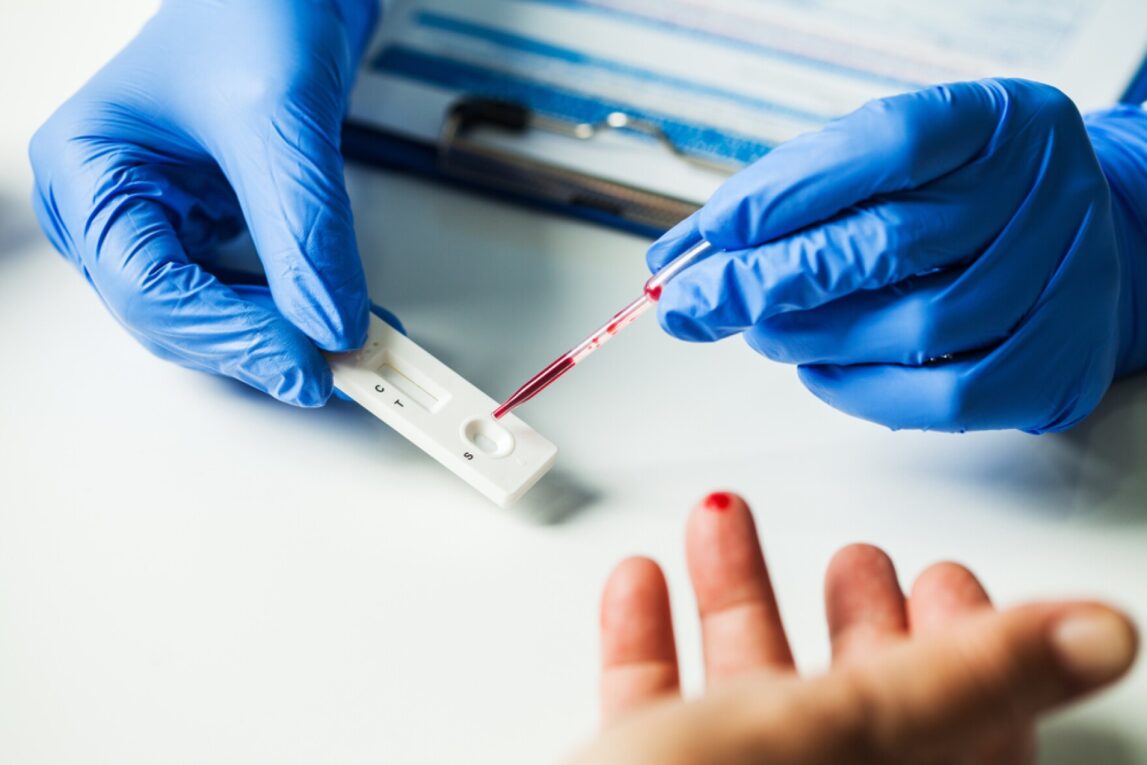
POCT refers to Rapid Diagnostics testing performed outside the conventional laboratory setting. With POCT, results are available more quickly because testing takes place near or at the site of patient care. Some examples of POCT used for rapid diagnostics include glucose monitoring, pregnancy testing, rapid influenza testing, and cardiac marker testing. POCT has several advantages over traditional lab testing including faster turnaround times, reduced costs from not needing sample transportation, and ability to test in alternate locations like clinics, pharmacies, and patient homes. However, POCT devices may not be as sensitive or specific as lab equipment. Regulatory oversight of POCT quality and performance is also important.
Lateral Flow Immunoassays
Lateral flow immunoassays, also known as immune-chromatographic assays, are a type of POCT that uses antibody-coated test strips to detect the presence or absence of analytes. They are used for conditions like infectious diseases, drugs of abuse, and pregnancy. A sample is applied to the test strip which uses capillary action to flow down the strip. Interactions between antibodies and antigens cause the development of visual lines that indicate a positive or negative result, usually within 10-30 minutes. Lateral flow assays are simple to use, require minimal training, and provide results quickly without instrumentation. However, sensitivity and specificity may be lower than lab-based methods.
PCR-Based Molecular Diagnostics
Polymerase chain reaction (PCR) is a rapid technique used to amplify trace amounts of DNA or RNA from biological samples. Real-time quantitative PCR (qPCR) allows for continuous monitoring of the amplification process, providing quantitative results. Isothermal amplification methods like loop-mediated isothermal amplification (LAMP) operate at a constant temperature and are simpler than qPCR. Molecular diagnostics based on PCR and other techniques allow detection of pathogens at the genetic level with high sensitivity and specificity. While current PCR instruments don’t satisfy true point-of-care criteria due to size, cost and technical complexity, portable battery-powered devices and integrated microfluidic cartridges are expanding PCR’s use outside conventional labs for rapid diagnosis.
Advancements in Immunoassay Technologies
More sensitive and specific immunoassays are advancing rapid diagnostics. Digital immunoassays count individual biomolecule targets in Nanoliter-sized wells, enabling detection of low analyte concentrations from small sample volumes. Microfluidic paper-based analytical devices (μPADs) provide simple and inexpensive assay platforms using patterned paper as the substrate. Isothermal multiple displacement amplification coupled to lateral flow strips detects antigens in under 30 minutes without equipment. Fluorescence-based assays detect multiple targets simultaneously with high resolution using smartphone cameras rather than test strip readers. Nanoparticle bioconjugates amplify signals for improved limits of detection. Advances like these are enhancing the accuracy, versatility and ease-of-use of immunoassays.
Innovations in Molecular Diagnostic Technologies
Molecular diagnostics are also rapidly progressing. Miniaturized real-time PCR chips integrate all assay components and fluidics on credit card-sized devices run by portable instruments. PCR results in under an hour are enabling use in emergency departments and ambulances. Sample-in-answer-out microfluidic diagnostic devices require only adding patient samples for fully automated molecular assays independent of user expertise.CRISPR-based molecular diagnostics utilize the adaptive immune system of bacteria to detect nucleic acids with exceptional specificity. Near-infrared fluorescence probes allow rapid multiplex detection in biologic samples using portable scanners. As molecular technologies continue integrating, miniaturizing and automating sample preparation and analysis, rapid accurate diagnosis from virtually any location will become feasible.
Rapid Testing in Disease Outbreaks and Epidemics
Rapid diagnostics have become essential for detecting, monitoring and controlling disease outbreaks and pandemics. During the 2014-2016 Ebola epidemic, lateral flow assays provided quick screening at care sites in West Africa. Near real-time PCR allowed dynamic transmission monitoring throughout affected regions. In 2020, rapid antigen tests played a crucial role in scaling up COVID-19 testing capacity globally. Molecular testing guided immediate isolation of cases and contact tracing to curtail viral spread. Rapid platforms for influenzas, noroviruses, bacterial infections and other emerging pathogens allow characterization of outbreak dynamics, enabling timely public health responses. As threats continue emerging, further innovation will be vital for rapid diagnosis enabling outbreak containment through faster clinical and epidemiological decision making.
The continuously expanding roles of rapid diagnostics exemplify ongoing revolutionizing of medical testing through technological innovation. Faster, more accurate and easier to use platforms are propelling diagnostic capabilities closer to the point-of-care and enabling new applications from screening to outbreak response. Further research promises significant enhancements including multiplex assays, lab-free formats, and ultimate integration into sustainable decentralized healthcare models. Rapid diagnostics will remain instrumental for improved patient management and global health security in the years to come.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it