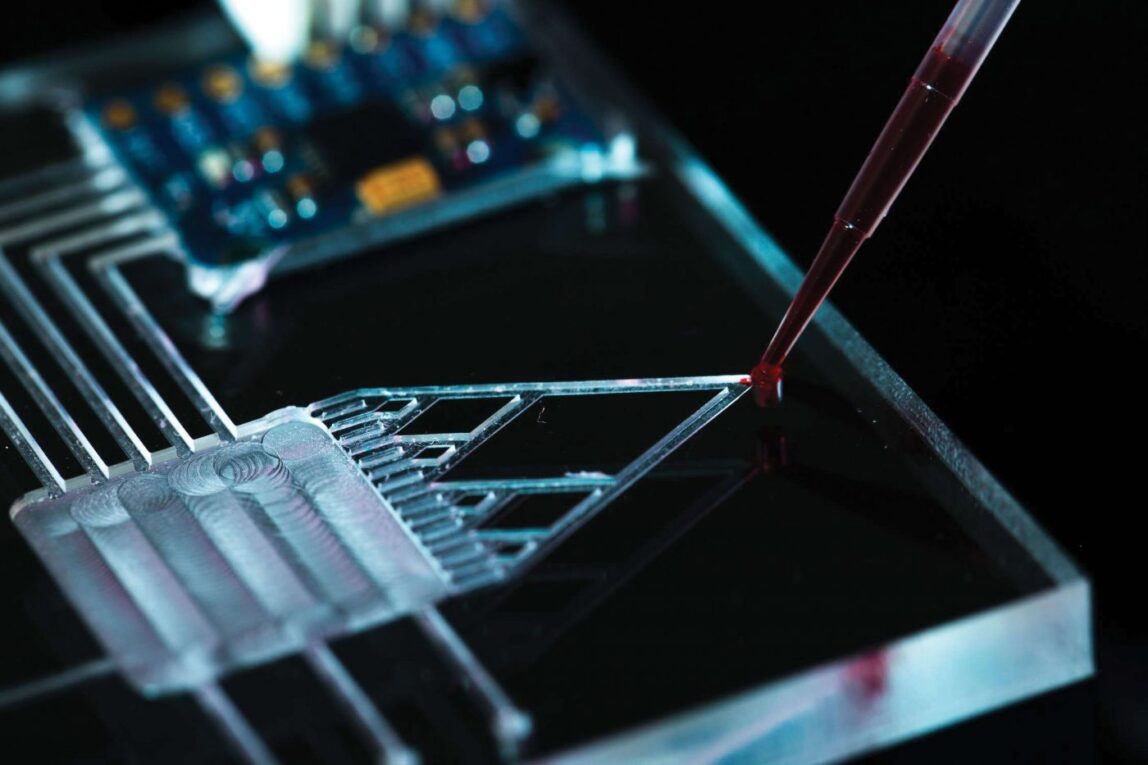
Microfluidic deals with the manipulation of fluid flows on the micrometre scale. The field has grown tremendously in the last few decades due to its applications in fields like biomedical diagnostics, drug discovery and chemical synthesis.
What are the key principles of Microfluidic?
At micro- and nano- scales, fluid behavior differs significantly from macro-scale. Some key principles governing microfluidic flows include:
Surface tension dominates over gravity – When dimensions are reduced to micrometers, surface forces like surface tension become dominant over body forces like gravity. This allows precise manipulation of liquids within narrow channels using surface energies.
Laminar flow regime – At small scales, viscous forces are much greater than inertial forces resulting in laminar or layered flows rather than turbulent mixing. Fluids flow smoothly in parallel layers with no disruptions between the layers.
Diffusion dominates over convection – At microscales, mass transfer occurs majorly via diffusion rather than conventional convection. This property is exploited for rapid mixing in microfluidic devices.
Miniaturized total analysis systems
One of the key advantages of Microfluidic is the ability to integrate and automate multiple fluidic operations on a single microchip. This has enabled development of miniaturized total analysis systems (μTAS) capable of performing complex biochemical analyses point-of-care. μTAS devices integrate sample preparation, chemical reactions, separations and detections on self-contained chips comparable in size to a credit card.
Some examples of μTAS systems include microfluidic ELISA chips for rapid pathogen detection from blood/urine samples and “micro labs-on-a-chip” that can quantitate biomarkers or metabolites from minute biofluid volumes. The reduced operation volumes, time and costs have made μTAS enormously useful for applications such as medical diagnostics, food/water safety testing and biothreat agent screening.
Droplet Microfluidic – compartmentalizing biomolecular experiments
One exciting area is the use of Microfluidic for generating, manipulating and analyzing discrete fluid droplets in an immiscible continuous phase. Monodisperse droplets as picoliter-femtoliter reaction chambers allow compartmentalizing molecules, cells or microbes for single-entity analysis.
Precisely controlling droplet generation, transportation, merging/mixing, sorting and detection has enabled high-throughput screening of cells/molecules, directed evolution experiments and synthesizing DNA/RNA/proteins in droplets. This “digital Microfluidic” approach mimics microplate assays but with greatly improved throughput, automation, and scale using much smaller sample/reagent volumes. Droplet Microfluidic is finding myriad applications from vaccines and biotherapeutics development to synthetic biology.
Organ-on-a-chip platforms for disease modeling and drug testing
A longstanding goal has been developing models that accurately simulate human physiology and pathology at an organ/whole-body level. Microfluidic organ-on-a-chip systems co-culture living cells on polymer/glass chips under continuously perfused fluid flow and mechanical forces resembling tissue-level microenvironments.
By recapitulating organ-level functions, such as gut-liver interactions, lungs-on-a-chip mimic airway-blood interfaces or blood-brain barrier chips, we can gain insights into complex disease mechanisms. These models are also being used to predict human pharmacokinetics and test new drugs/therapies in relevant disease settings more accurately than conventional models. Though still in early stages, organ chips promise safer, faster and more predictive preclinical research as well as personalized medicine applications.
Microfluidic in biomanufacturing and synthetic biology
Continuous biomanufacturing using microfluidic platforms offers significant improvements over batch fermentation methods. Integrated cell culture, reactions, separations and measurements facilitate highly controlled and automated processes leveraging laminar flows, rapid mixing and adjustable culture conditions.
Combining Microfluidic with synthetic biology has led to novel bio-factories where microbial cells are programmed to synthesize products of interest. By integrating cell-free gene circuits that sense and respond to environmental triggers, one can develop smart, on-demand production systems. Some envision scaling-out such continuous biomanufacturing systems for production of biologics, industrial enzymes and specialty chemicals.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it